.png?width=2000&height=714&name=Pichia%20pastoris%20-%20plate%20with%20cells%20(1).png)
Pichia pastoris
Pichia pastoris is a methylotrophic yeast species (it can use methanol as a source for energy) that has become a popular protein expression system. Several advantages make it an ideal host for protein expression, e.g.;
- The production of large amounts of recombinant protein
- Post-translational modifications of proteins
- The secretion of proteins into the culture medium
Pichia can be grown in minimal media to high cell densities and is easily genetically manipulated, making it an economical choice for protein expression. Overall, Pichia pastoris is a versatile, efficient, and reliable protein expression system.
Protein Expression
Protein expression refers to the production of a functional protein from its genetic code. In microorganisms such as bacteria, yeast, and fungi, protein expression is an important tool for the production of therapeutic proteins, vaccines, enzymes, and other products. However, there are several challenges that can affect the efficiency and yield of the process.
Defining the most suitable expression system and procedure is crucial to optimize the yield and quality of the respective target protein.
Want To Hear The Story First-Hand?
Dr. Christina Dickmeis explains how she improved her process of protein production in Pichia pastoris in an exclusive interview on sbi's Lab Chats Episode 1.
Methanol Induction
Methanol induction is commonly applied and a critical step in the expression of recombinant proteins using the Pichia pastoris expression system. The used promotor is an inducible promoter that drives the expression of genes in the presence of methanol.
By inducing with methanol, the cultivation is divided into two phases:
- The cell growth phase, with ideal conditions for biomass production
- The protein production phase, that focuses on efficient protein generation
Methanol induction is usually performed after the yeast cells have reached a high cell density in the growth phase. The yeast cells are then exposed to a certain concentration of methanol and incubated for a period of time to induce the expression of the recombinant protein. The level of methanol in the induction medium and the timing of the addition can be optimized for different recombinant proteins to achieve the highest expression levels. Finally, the yeast cells are harvested, and the recombinant protein is purified using standard protein purification techniques.
Challenges of Methanol Induction
Certain levels of methanol (>1%) are toxic and reduce biomass formation
![]() Methanol is also used as substrate and induces metabolic changes
Methanol is also used as substrate and induces metabolic changes
![]() The right time of induction is crucial to achieve optimal protein yields
The right time of induction is crucial to achieve optimal protein yields
![]() Low but constant methanol levels are desirable for ongoing expression
Low but constant methanol levels are desirable for ongoing expression
![]() Methanol is harmful for humans and needs to be handled with care
Methanol is harmful for humans and needs to be handled with care
The Technology
Dr. Christina Dickmeis, a Senior Research Scientist at sbi, utilized biomass-based feeding to initiate methanol induction at the optimal time - once cell growth on the primary substrate (glycerol) was completed and sufficient biomass was generated, indicated by a reduction in growth rate.
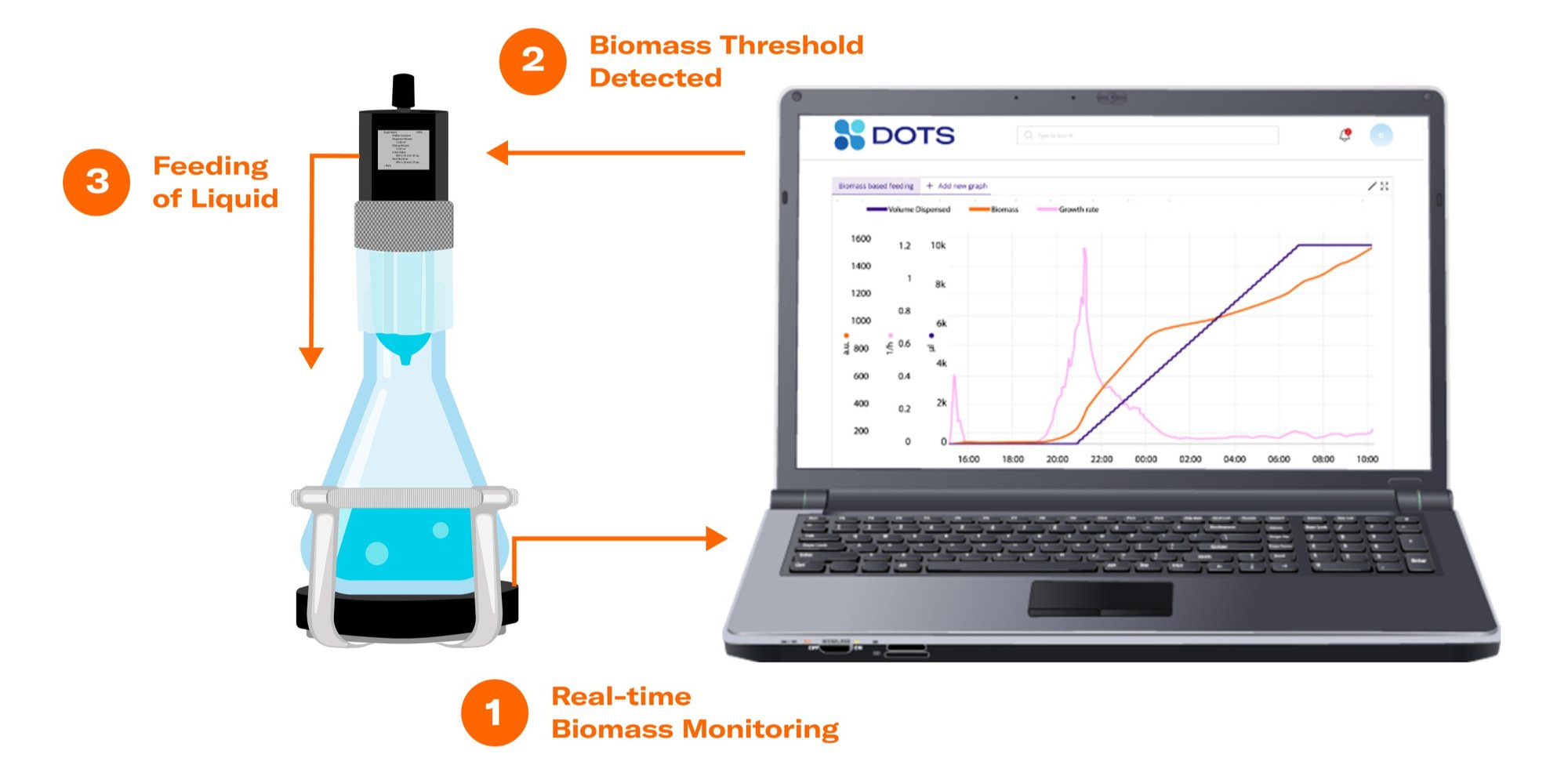
Biomass-based feeding in shake flasks increases the control options of bioprocesses carried out in this vessel type. The term describes the feeding of a substance to a growing microbial culture, initiated by the reach of a certain biomass level or growth rate. The automated substance release is enabled by the interconnectivity of the Cell Growth Quantifier (CGQ), a reliable biomass monitoring sensor, the state-of-the-art Liquid Injection System (LIS), and the powerful DOTS Software.
Biomass-based Feeding: Methanol Induction with Pichia pastoris
The methanol feed was started automatically, once the growth rate dropped below the threshold of 0.002 h-1.
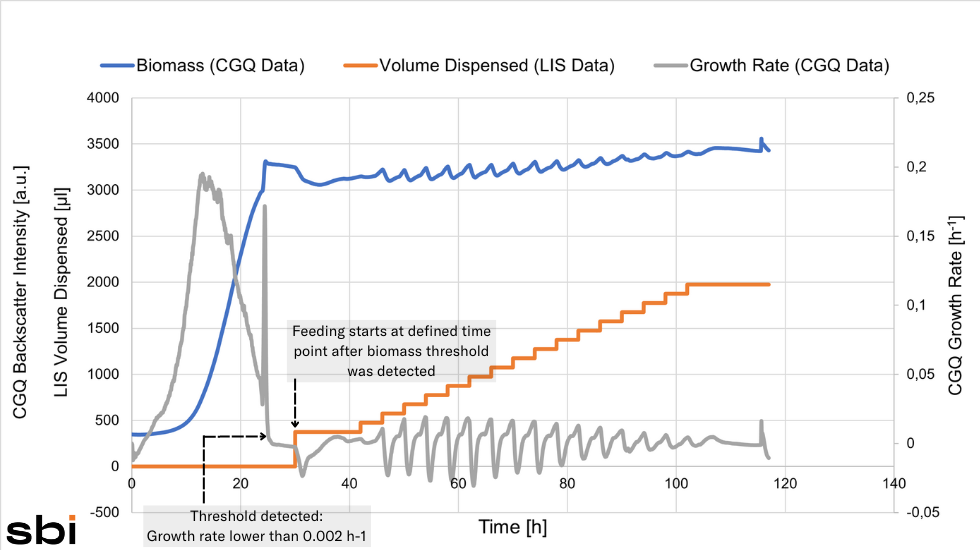
Testing Different Feeding Strategies
Christina tested different feeding profiles to find out which methanol induction strategy would result in the highest protein yield. Her data showed that Feeding Profile 2 was the most beneficial - a Single Shot of methanol followed by a pause in which the cells adapted to the new substrate and a subsequent Constant Feed.
.png?width=2000&height=691&name=Pichia%20-%20feeding%20profiles%201-3%20(1).png)
.png?width=2000&height=1127&name=Pichia%20%20-%20protein%20yields%20of%20diff%20feeding%20profiles%20(1).png)
Results
By implementing automated procedures, including biomass-based feeding, and further optimizing process conditions, the yield of active protein could be improved by a factor of 20, compared to Christinas first experiments with manual handling setups. Additionally, when comparing an automated with a manually performed Multi Shot experiment, manual handling requires approximately 2 hours and 40 minutes more time per experiment than the automated biomass-based feeding setup.
.png?width=80&height=80&name=increase-graphic%20(1).png)
20x
Increase of Active Protein Yield

9x
Faster Than Manual Sampling
.png?width=100&height=51&name=sbi%20logo%20(npws).png)
What's Next?
Christina will consolidate the data from her shake flask experiments and identify the conditions that have shown to bring the most favorable outcome. She will then use this info to set up a defined bioprocess in a bioreactor, scaling up her experiments and protein yields.The implementation of biomass-based feeding in shake flasks and the continuous online acquisition of data from different experiment runs provided critical information that helped Christina to further characterize her bioprocess with an improved outcome.
Having optimized the protein production phase, Christina wants to have a closer look on the cell growth phase next. Generating higher biomass levels of the yeast will likely enhance the final protein yields further.
From Estimation To High-Resolution Growth Curves
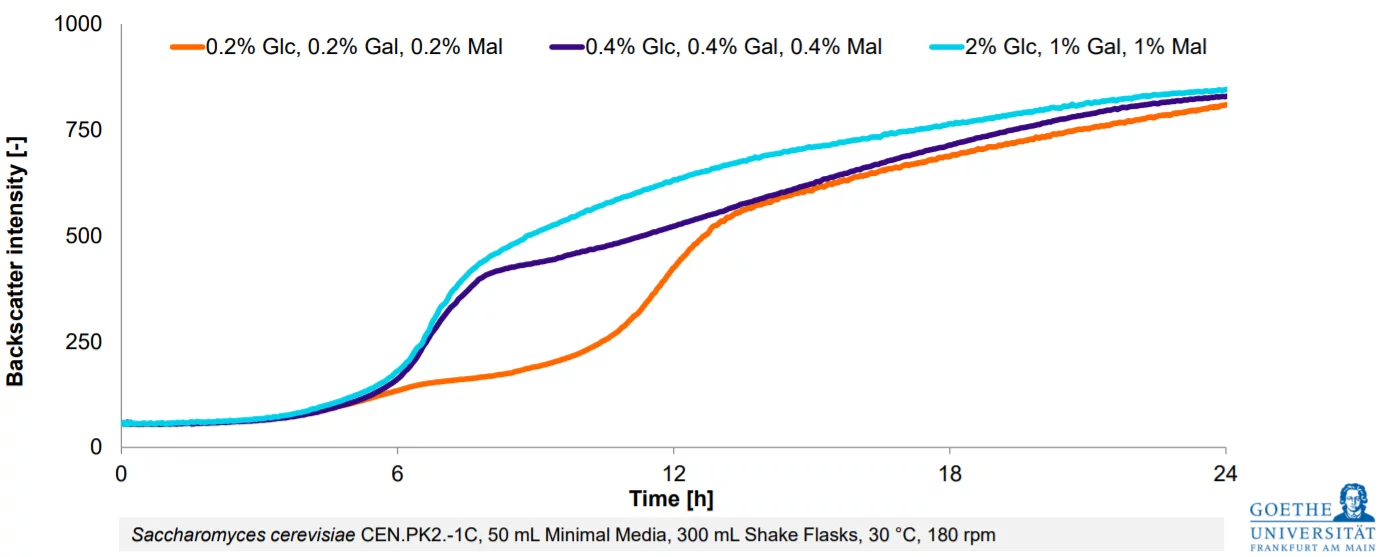
Customer Success Stories
.png)
-Kitana Manivone Kaiphanliam (Washington State University)

Want Results Like These?
We will work with you on a solution that works best for your application.
%20(1)%20(1).png)
